![Cover Picture: [Fe(H2O)5(NO)]2+, the “Brown‐Ring” Chromophore (Angew. Chem. Int. Ed. 25/2019) - Monsch - 2019 - Angewandte Chemie International Edition - Wiley Online Library Cover Picture: [Fe(H2O)5(NO)]2+, the “Brown‐Ring” Chromophore (Angew. Chem. Int. Ed. 25/2019) - Monsch - 2019 - Angewandte Chemie International Edition - Wiley Online Library](https://onlinelibrary.wiley.com/cms/asset/994a8c38-95fb-4e9e-a4c6-43434d2ce00c/anie201905910-toc-0001-m.jpg)
Cover Picture: [Fe(H2O)5(NO)]2+, the “Brown‐Ring” Chromophore (Angew. Chem. Int. Ed. 25/2019) - Monsch - 2019 - Angewandte Chemie International Edition - Wiley Online Library
Theoretical Study of Oxovanadium(IV) Complexation with Formamidoximate: Implications for the Design of Uranyl-Selective Adsorben
SOLVED: How many unpaired electrons is VO(H2O)5 expected to have and how could it be proven experimentally?
![Orientations of the VO²⁺ ions and [VO(H2O)5]²⁺ complexes in the ZAPH... | Download Scientific Diagram Orientations of the VO²⁺ ions and [VO(H2O)5]²⁺ complexes in the ZAPH... | Download Scientific Diagram](https://www.researchgate.net/publication/286004505/figure/fig3/AS:1132193107722246@1646947280502/Angular-variation-of-EPR-line-positions-of-VO-ZAPH-in-the-plane-a-ab-b-bc-and-c_Q320.jpg)
Orientations of the VO²⁺ ions and [VO(H2O)5]²⁺ complexes in the ZAPH... | Download Scientific Diagram

Effect of the solvent on the formation of new oxovanadium(IV) complexes with pentafluorobenzoate anions and 1,10-phenanthroline - ScienceDirect
![SOLVED: B – Complexes Complex Ion Coordination Number Molecular Geometry Name [VO2(H2O)3]+ [VO(H2O)5]2+ [V(H2O)6]3+ Analysis 1. Write a balanced redox equation for each conversion using Zn as the reducing agent. a) VO2+ SOLVED: B – Complexes Complex Ion Coordination Number Molecular Geometry Name [VO2(H2O)3]+ [VO(H2O)5]2+ [V(H2O)6]3+ Analysis 1. Write a balanced redox equation for each conversion using Zn as the reducing agent. a) VO2+](https://cdn.numerade.com/ask_previews/c07def3e-6d56-40ff-a5e3-203dd65cb17f_large.jpg)
SOLVED: B – Complexes Complex Ion Coordination Number Molecular Geometry Name [VO2(H2O)3]+ [VO(H2O)5]2+ [V(H2O)6]3+ Analysis 1. Write a balanced redox equation for each conversion using Zn as the reducing agent. a) VO2+
![SOLVED: Text: Question 3 A - Oxidation states Vanadium Species VO2+ V3+ Oxidation State Colour Complex Ion [VO2(H2O)4]+ VO(H2O)5]2+ [V(H2O)6]3+ B - Complexes Complex Ion Coordination Number Molecular Geometry Name [VO2(H2O)4]+ [VO(H2O)5]2- [ SOLVED: Text: Question 3 A - Oxidation states Vanadium Species VO2+ V3+ Oxidation State Colour Complex Ion [VO2(H2O)4]+ VO(H2O)5]2+ [V(H2O)6]3+ B - Complexes Complex Ion Coordination Number Molecular Geometry Name [VO2(H2O)4]+ [VO(H2O)5]2- [](https://cdn.numerade.com/ask_images/68c789418d3e4f1aabcb5b353a4e4c33.jpg)
SOLVED: Text: Question 3 A - Oxidation states Vanadium Species VO2+ V3+ Oxidation State Colour Complex Ion [VO2(H2O)4]+ VO(H2O)5]2+ [V(H2O)6]3+ B - Complexes Complex Ion Coordination Number Molecular Geometry Name [VO2(H2O)4]+ [VO(H2O)5]2- [
A Coordination Chemistry Study of Hydrated and Solvated Cationic Vanadium Ions in Oxidation States +III, +IV, and +V in Solution
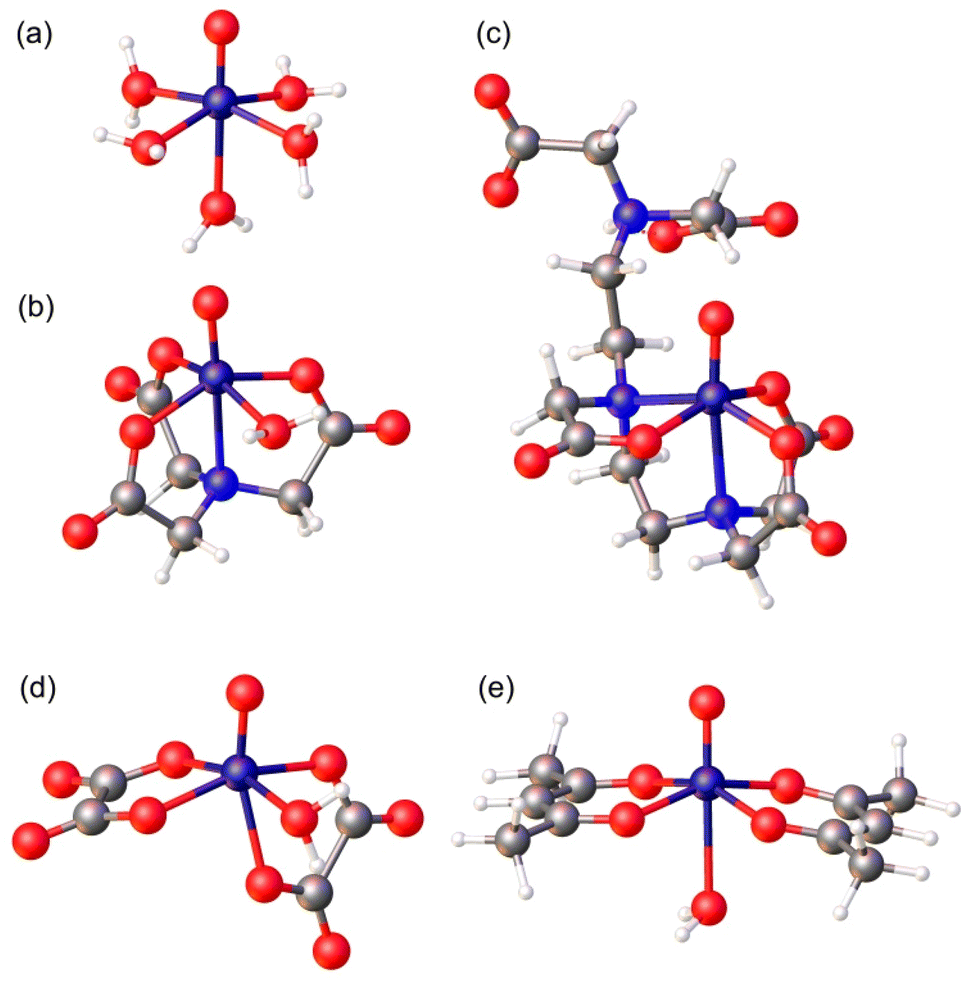
Magnetic and relaxation properties of vanadium( iv ) complexes: an integrated 1 H relaxometric, EPR and computational study - Inorganic Chemistry Frontiers (RSC Publishing) DOI:10.1039/D2QI02635J
![SOLVED: B – Complexes Complex Ion Coordination Number Molecular Geometry Name [VO2(H2O)3]+ [VO(H2O)5]2+ [V(H2O)6]3+ Analysis 1. Write a balanced redox equation for each conversion using Zn as the reducing agent. a) VO2+ SOLVED: B – Complexes Complex Ion Coordination Number Molecular Geometry Name [VO2(H2O)3]+ [VO(H2O)5]2+ [V(H2O)6]3+ Analysis 1. Write a balanced redox equation for each conversion using Zn as the reducing agent. a) VO2+](https://cdn.numerade.com/ask_previews/c07def3e-6d56-40ff-a5e3-203dd65cb17f.gif)
SOLVED: B – Complexes Complex Ion Coordination Number Molecular Geometry Name [VO2(H2O)3]+ [VO(H2O)5]2+ [V(H2O)6]3+ Analysis 1. Write a balanced redox equation for each conversion using Zn as the reducing agent. a) VO2+

Processes | Free Full-Text | A Facile Hydrothermal Synthesis of S-VO2-Cellulose Nanocomposite for Photocatalytic Degradation of Methylene Blue Dye

Insights on the mechanistic features of catalytic oxidations of simple and conjugated olefins promoted by VO(acac)2/H2O2 system, in acetonitrile: A computational study - ScienceDirect
![Orientations of the VO²⁺ ions and [VO(H2O)5]²⁺ complexes in the ZAPH... | Download Scientific Diagram Orientations of the VO²⁺ ions and [VO(H2O)5]²⁺ complexes in the ZAPH... | Download Scientific Diagram](https://www.researchgate.net/publication/286004505/figure/fig2/AS:1132193107722244@1646947280476/EPR-spectrum-of-a-VO-doped-ZAPH-single-crystal-with-the-magnetic-field-at-0-from-the_Q320.jpg)
Orientations of the VO²⁺ ions and [VO(H2O)5]²⁺ complexes in the ZAPH... | Download Scientific Diagram
![Molecular formulas of (1) [VO(dipic)(H2O)2]•2 H2O, (2) [VO(dipic)(bipy... | Download Scientific Diagram Molecular formulas of (1) [VO(dipic)(H2O)2]•2 H2O, (2) [VO(dipic)(bipy... | Download Scientific Diagram](https://www.researchgate.net/publication/361558060/figure/fig1/AS:1175627562532865@1657302861975/Molecular-formulas-of-1-VOdipicH2O22-H2O-2-VOdipicbipy-VOidabipy2.png)
Molecular formulas of (1) [VO(dipic)(H2O)2]•2 H2O, (2) [VO(dipic)(bipy... | Download Scientific Diagram
![Orientations of the VO²⁺ ions and [VO(H2O)5]²⁺ complexes in the ZAPH... | Download Scientific Diagram Orientations of the VO²⁺ ions and [VO(H2O)5]²⁺ complexes in the ZAPH... | Download Scientific Diagram](https://www.researchgate.net/publication/286004505/figure/fig5/AS:1132193107705862@1646947280563/EPR-spectrum-n95GHz-of-the-powdered-sample-of-VOZAPH-at-room-temperature-a_Q320.jpg)
Orientations of the VO²⁺ ions and [VO(H2O)5]²⁺ complexes in the ZAPH... | Download Scientific Diagram