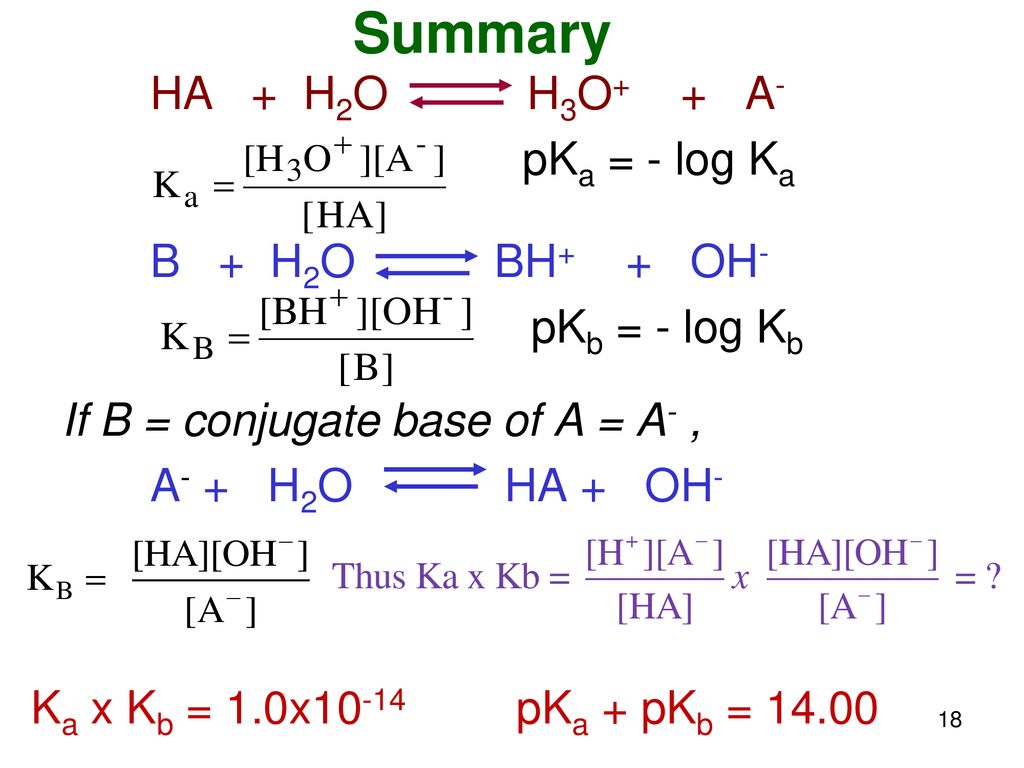
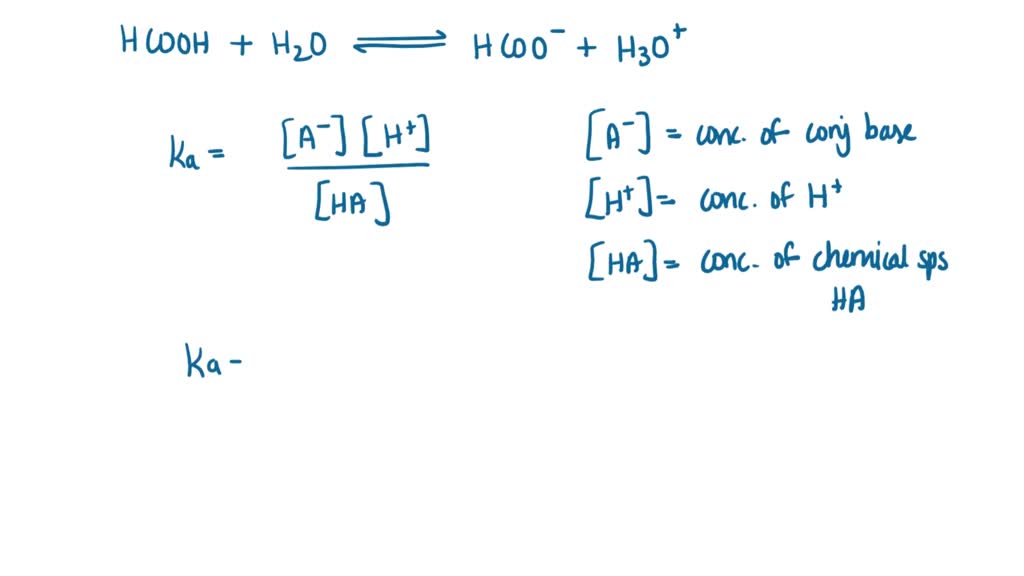54. If H2 + 1/2 02- >H20, AH=- 68.39 kcal K + H2O → KOH (aq) + 1/2 H2, AH=- 48 kcal KOH + water ->KOH (aq), AH=- 14 kcal The heat of formation of KOH is (A) - 68.39 +48 – 14 (B) - 68.39 – 48 + 14 (C) 68.39 – 48 + 14 (D) + 68.39 +48 – 14

Water-exchange rate constants, k H2O , for a particular water molecule... | Download Scientific Diagram
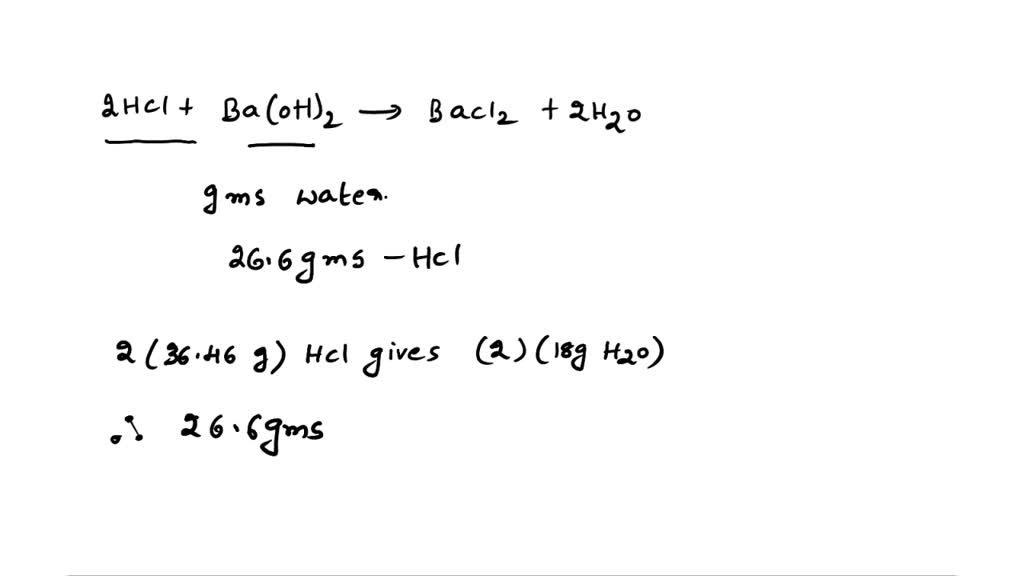
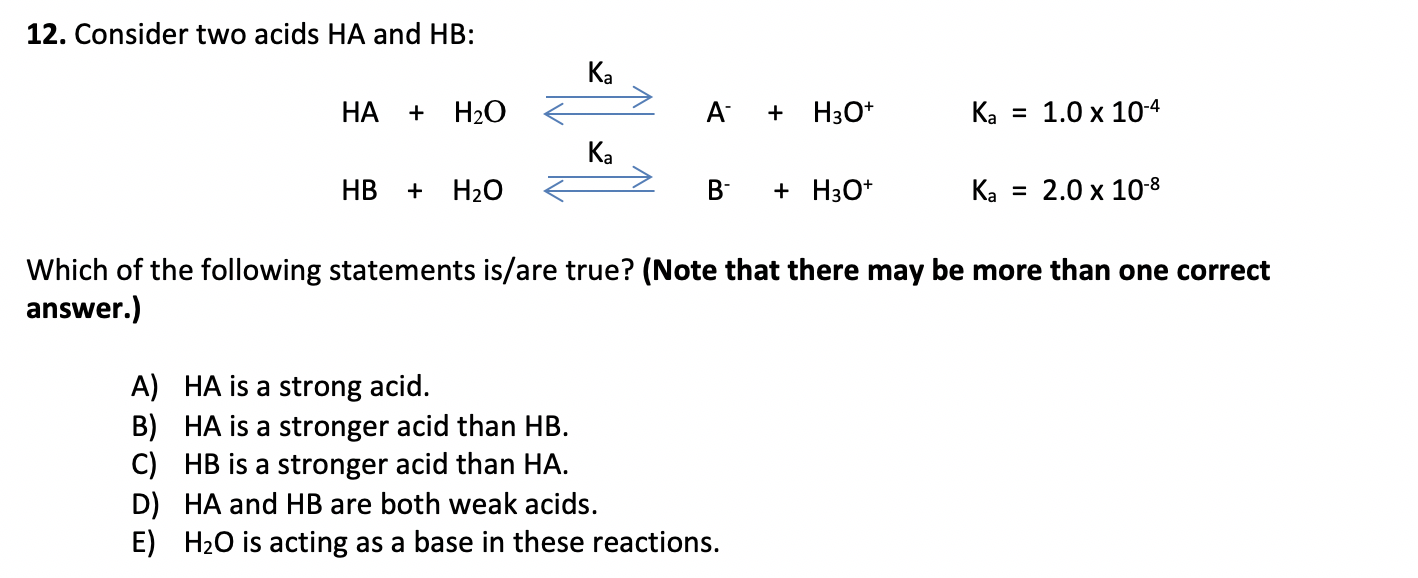
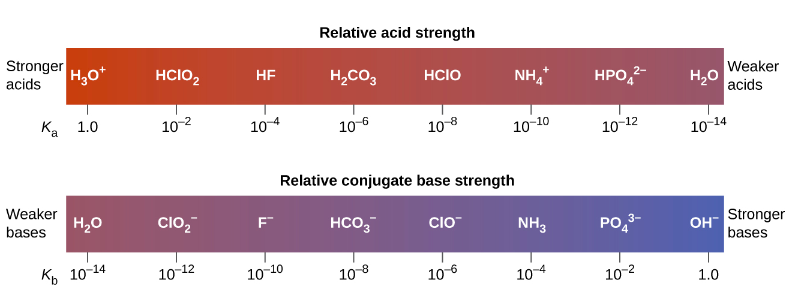
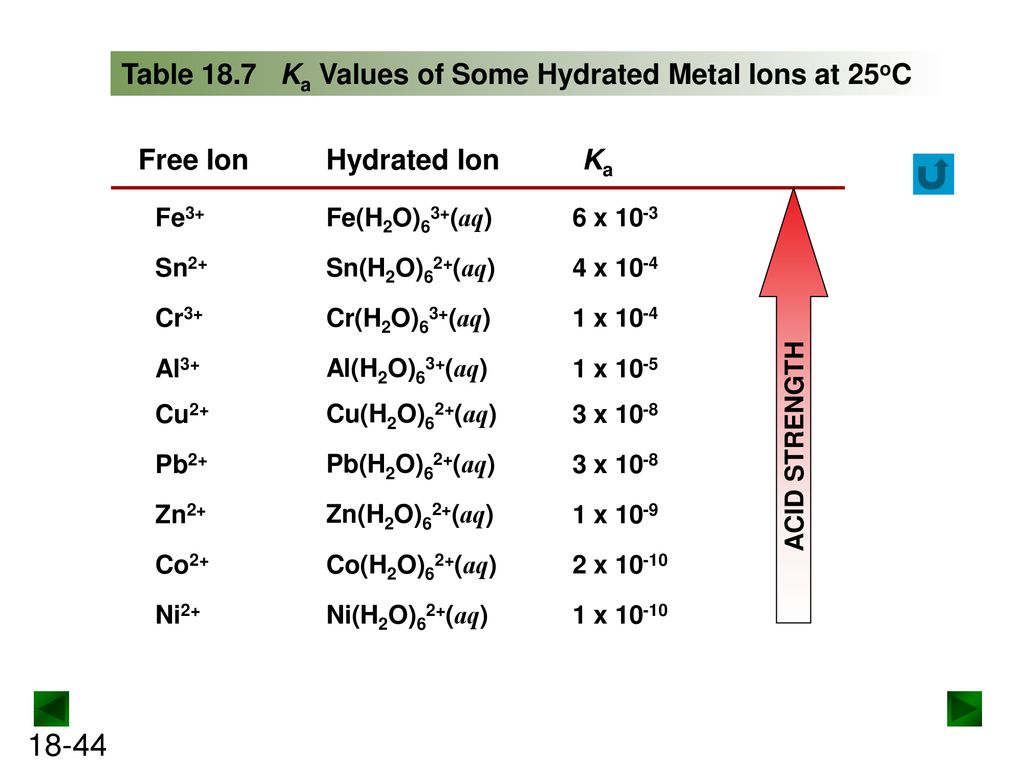
SOLVED: The equilibrium constant Ka for the reaction Co(H2O)6^3+ (aq) + H2O (l) —> Co(H2O)5(OH)2+ (aq) + H3O+ (aq) is 1.0x10^-5 A) Calculate the pH of a 0.16 M solution of Co(H2O)6^3+.

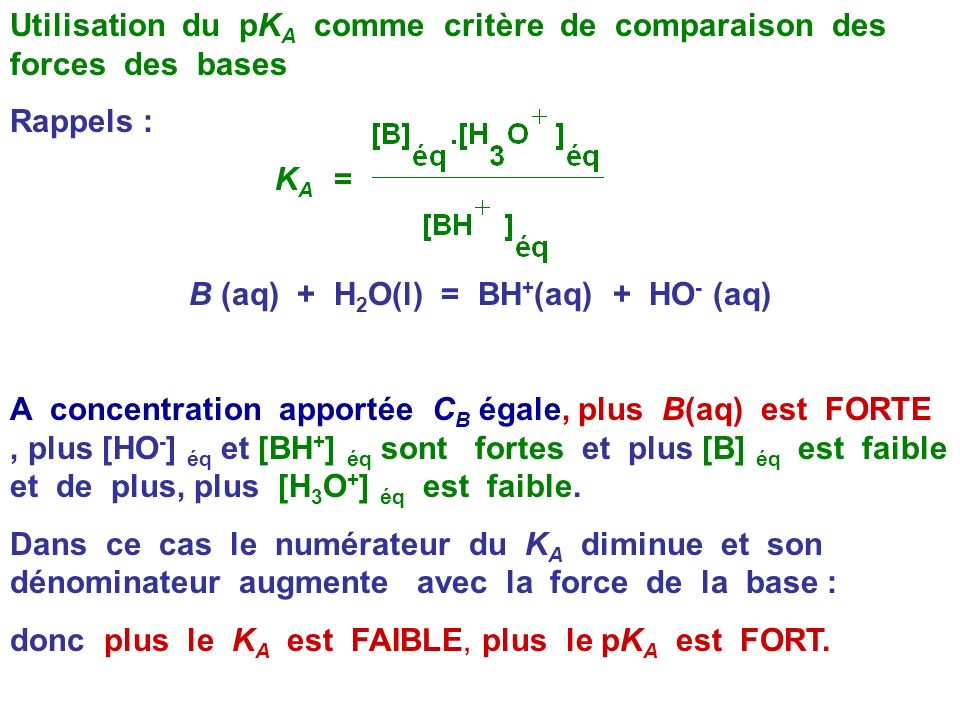
SOLVED: Ka: SO4^2- (aq) + H2O (l) ⇌ HSO4- (aq) + OH- (aq) Kb: HSO3- (aq) + H2O (l) ⇌ H2SO3 (aq) + OH- (aq)
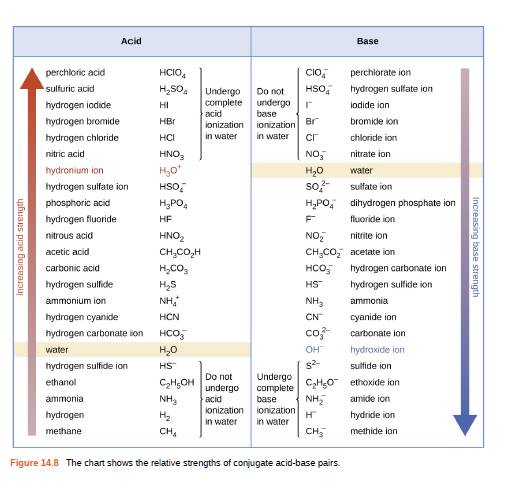
Using the K a value of 1.4 × 10 − 5 , place Al(H 2 O) 6 3 + in the correct location in Figure 14.8. | bartleby

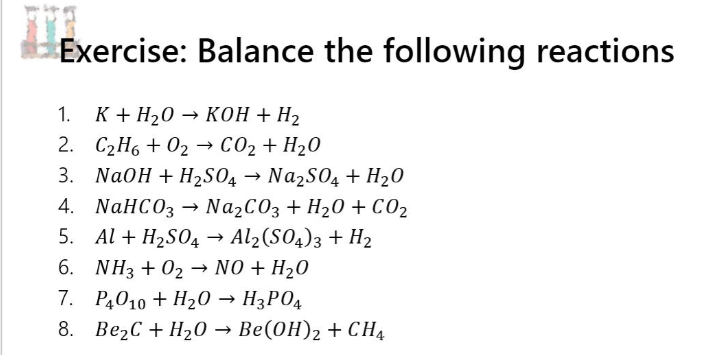
Oxidation Number method. K+H2O=KOH+H2. Balance the chemical equation by oxidation Number method. - YouTube