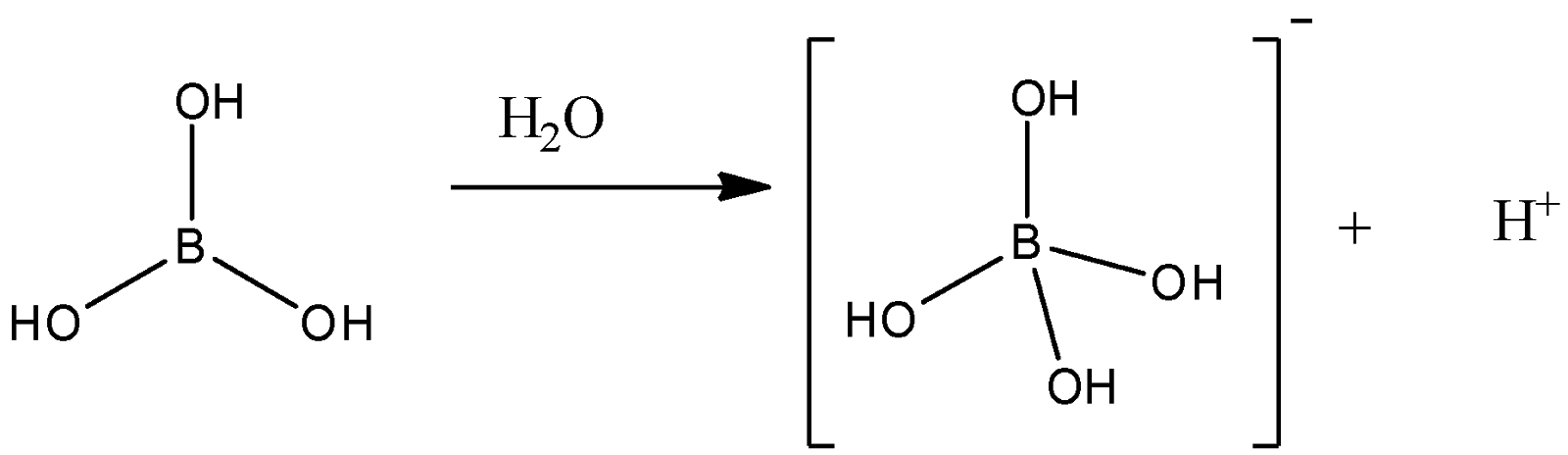
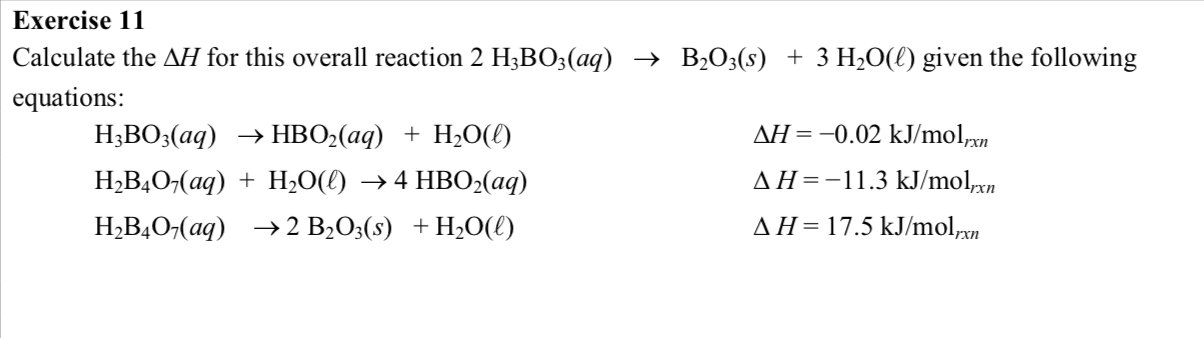
Experimental determination and thermodynamic modeling of solid–liquid equilibria in the system NaCl–Na2SO4–H3BO3–H2O at 323.15 K and its application in industry - ScienceDirect

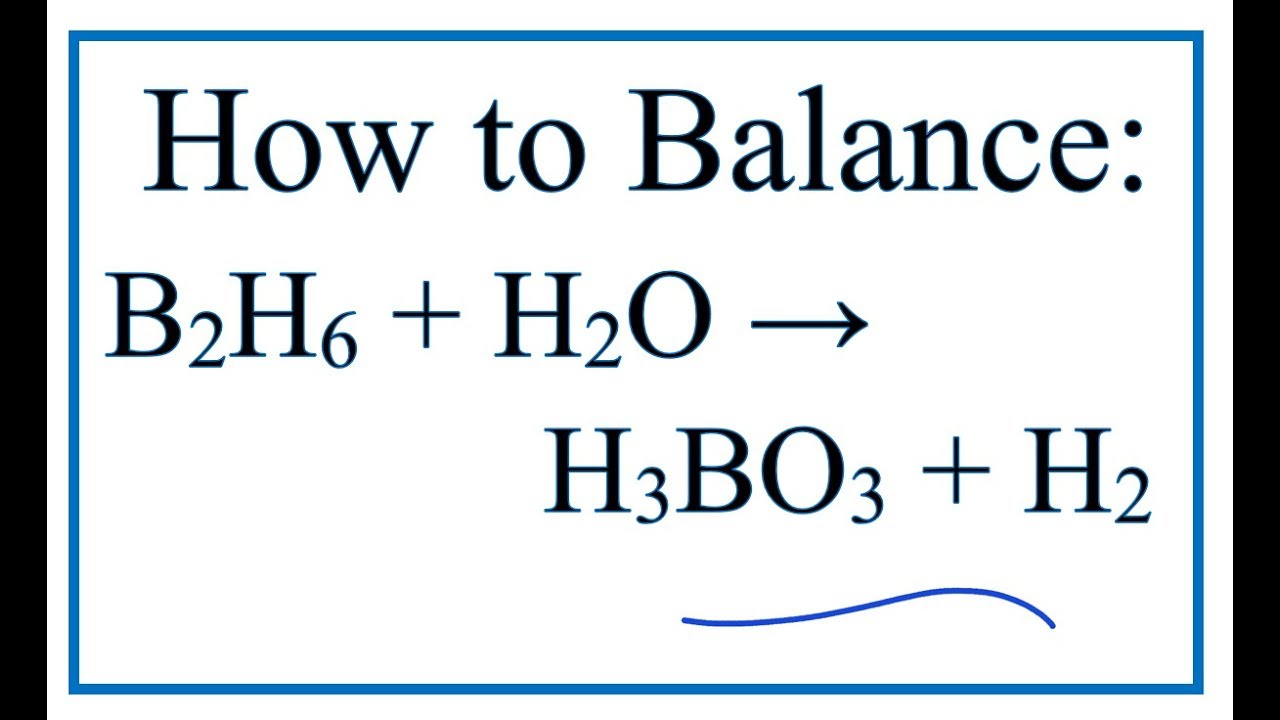
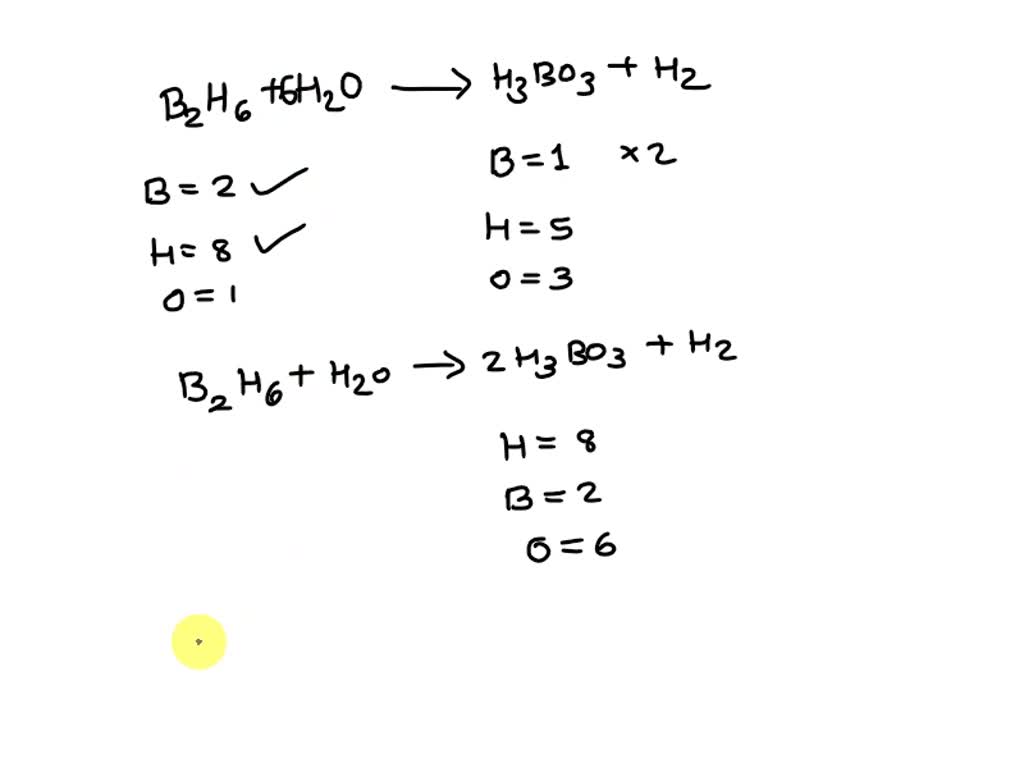
B2O3 + 3 H2O ---> 2 H3BO3 If diboron trioxide is reacted with water, the product is boric acid. What mass of boric acid is obtained from the full reaction. - ppt video online download
![Write the reaction of action of heat on boric acid [H3BO3]? - Find 2 Answers & Solutions | LearnPick Resources Write the reaction of action of heat on boric acid [H3BO3]? - Find 2 Answers & Solutions | LearnPick Resources](https://www.learnpick.in/files/answerimages/5eb22de957ba09459f6fe1c23b3b4438.jpg)
Write the reaction of action of heat on boric acid [H3BO3]? - Find 2 Answers & Solutions | LearnPick Resources

B2O3 + 3 H2O ---> 2 H3BO3 If diboron trioxide is reacted with water, the product is boric acid. What mass of boric acid is obtained from the full reaction. - ppt video online download

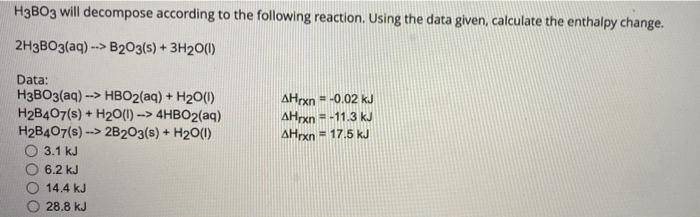
H3B03 on heating decomposes in two ways: 1. HBO3 + HBO2 + H2O II. H2B03 → B2O3 + H2O If 9 moles of H3BO3 are taken, some part decomposed like (1) and

SOLVED: How many grams of H3BO3 are produced from 124 g of H2O in the following reaction? BF3(g) + 3 H2O (l) -> 3 HF(aq) + H3BO3(aq) 142 g 70.9 g 47.3 g 425 g